What causes an LED to turn blue?
Modern lighting, displays, and electronics have been completely transformed by Light-Emitting Diodes (LEDs), which provide energy efficiency, extended lifespans, and versatility that conventional incandescent or fluorescent bulbs cannot match. Blue light has emerged as one of the most common colours produced by LEDs, and it powers everything from LED headlights to smartphone screens to even medical equipment. However, what specifically triggers the blue light that an LED emits? The materials utilised in their manufacture, deliberate technical decisions, and the basic physics of LED operation all hold the key to the solution. In order to comprehend this phenomenon, we must first dissect the light-generating process of LEDs and then look at the particular elements that cause their output to lean toward the blue portion of the electromagnetic spectrum.

Fundamentally, LEDs are semiconductor devices that use a process known as electroluminescence to generate light. LEDs produce light when electrons and "holes" (positive charge carriers) recombine within a semiconductor material, as opposed to incandescent bulbs, which produce light by heating a filament-a wasteful process that loses the majority of energy as heat. This is how it operates: Electrons from the negatively charged "n-type" semiconductor cross a junction into the positively charged "p-type" semiconductor when an electric current is provided to the LED. These electrons release energy in the form of photons, or particles of light, as they strike and fill the holes in the p-type material. The semiconductor's band gap energy determines the hue of this light; the greater the band gap (the energy differential between the semiconductor's valence band, which contains holes, and conduction band, which contains electrons), the shorter the wavelength of the light that is released. LEDs that create blue light need semiconductors with a relatively broad band gap because blue light has a short wavelength (450–495 nanometres). The primary and most important factor influencing the emission of blue light is this material attribute.
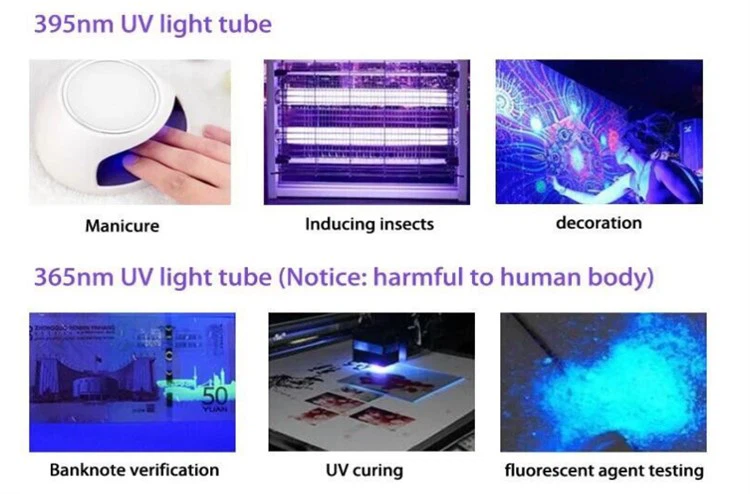
The creation of semiconductors based on gallium nitride (GaN) and related alloys, including indium gallium nitride (InGaN), was the major advancement in blue LED technology, which was acknowledged with the 2014 Nobel Prize in Physics. Because typical semiconductor materials (such as gallium arsenide, which is used for red and green LEDs) have too small a band gap to produce short-wavelength blue light, scientists had difficulty developing effective blue LEDs prior to the 1990s. On the other hand, GaN has a broad band gap of roughly 3.4 electron volts (eV), which is exactly the energy required to emit ultraviolet (UV) light. Engineers can lower the band gap by incorporating tiny amounts of indium into GaN to create InGaN. This shifts the output light from ultraviolet to blue by lowering the band gap energy. For instance, light with a wavelength of about 450 nm is emitted by an InGaN semiconductor with a band gap of about 2.7 eV, making it ideal for brilliant blue illumination. Because InGaN can be alloyed to adjust the band gap, it has become the standard material for blue LEDs. Blue LEDs (and the white LEDs that depend on them) would not be possible without GaN-based semiconductors.
The LED's quantum well structure is another crucial component that permits the production of blue light. A thin layer of semiconductor (usually InGaN) positioned between two thicker layers of another semiconductor (usually GaN itself) is called a quantum well. The electrons and holes inside the InGaN layer are restricted, or "trapped", in a way that changes their energy levels because the layer is so thin-typically only a few nanometres thick. The efficiency of the LED is increased by this confinement, which raises the likelihood that electrons and holes will recombine and produce photons. The thickness and composition of the quantum well are carefully regulated for blue LEDs; a narrower well or a larger indium concentration can fine-tune the emission wavelength to the required blue range. For example, light may shift to 470 nm from a 3-nanometre-thick InGaN quantum well with 20% indium content and 460 nm from a 5-nanometre well with 15% indium. Blue LEDs are sufficiently bright for practical applications, such as high-power LED floodlights and indicator lights on electronics, thanks to quantum wells' ability to lessen non-radiative recombination, which is the loss of energy as heat rather than light.
Blue light can also be an unexpected result of LEDs, most notably white LEDs, even though many LEDs are made specifically to create it. The majority of white LEDs employ a "phosphor conversion" technique, in which a blue LED chip is coated with a yellow phosphor material (typically cerium-doped yttrium aluminium garnet, or YAG:Ce), since white light cannot be directly produced by a single semiconductor (since it requires a mix of wavelengths across the visible spectrum). A portion of the blue light from the LED is absorbed and reemitted as yellow light when it strikes the phosphor. To the human sight, the remaining blue light appears as white light because it blends with the yellow light. Not all blue light is transformed, though, if the phosphor coating is uneven, excessively thin, or of low quality. This can produce a "cool white" or "blue-tinted" glow, which is typical of inexpensive LED bulbs or old fixtures with phosphor that has deteriorated over time. Because blue light affects the generation of melatonin, excessive blue light from white LEDs can occasionally induce eye strain or interfere with circadian rhythms. This emphasises the significance of appropriate phosphor design. This unexpected blue light is caused by poor phosphor integration rather than a defect in the LED's fundamental functionality.
Although they don't "cause" the LED to create blue light in the first place, environmental conditions can also affect how intense or how an LED seems to emit blue light. The band gap of the semiconductor may widen significantly when LEDs heat up (a common problem in high-power applications), moving the emission wavelength toward the red end of the spectrum. This is one example of how temperature impacts LED performance. This could result in a little change in wavelength for blue LEDs from 450 nm to 455 nm, which is barely perceptible to the naked eye but quantifiable with instruments. On the other hand, some high-performance LEDs (such as those found in projectors) have cooling systems since running them at lower temperatures can improve their efficiency and output of blue light. Current density is another consideration. While a blue LED's brightness can be increased by raising its electric current, an excessive current can result in "efficiency droop," or a decrease in light output per unit of current. Excessive current in extreme situations can harm the structure of the quantum well, resulting in either total failure or a permanent color shift that includes enhanced emission of blue light. Although these external conditions can alter an LED's performance over time, they do not alter the LED's intrinsic capacity to create blue light.
In conclusion, the three main causes of blue light emission from LEDs are the semiconductor material's band gap energy, the application of GaN-based alloys (such as InGaN) that permit short-wavelength light, and the quantum well structure that improves efficiency and adjusts the emission wavelength. While unwanted blue light (as in certain white LEDs) results from phosphor-related problems, intentionally designed blue LEDs use similar principles to provide brilliant, efficient blue light for particular applications. Although they can have an impact on performance, environmental conditions such as temperature and current do not change the fundamental mechanism of blue light emission. Knowing these reasons not only clarifies the existence of blue LEDs but also draws attention to the engineering advancements that enabled them, advancements that still propel lighting, displays, and renewable energy forward. Researchers are looking into new materials (such as aluminum gallium nitride for deeper blue or UV light) and designs to increase the efficiency of blue LEDs as LED technology advances. This could lead to new applications in medical therapy, water purification, and next-generation displays.
FAQs
Q1. How Can I Get This Samples?
A1: Hi, easy for this.give me your address and tell me which item you need,we will arrange sent to you by DHL or FedEx.
Q2:How About Your Quality?
A2: All raw material with top quality to ensure high luminous and enough brightness.
Q3:What About The Lead Time?
A3:Sample needs 3-5 days, mass production time needs 25-40 days after receving the deposit
Shenzhen Benwei Lighting Technology Co.,Ltd
Telephone: +86 0755 27186329
Mobile(+86)18673599565
Whatsapp :19113306783
Email:bwzm15@benweilighting.com
Skype: benweilight88
Web: www.benweilight.com




